Your new post is loading...

|
Scooped by
Beeyond
|
Mitigating the foreign body response (FBR) to implantable medical devices (IMDs) is critical for successful long-term clinical deployment. The FBR is an inevitable immunological reaction to IMDs, resulting in inflammation and subsequent fibrotic encapsulation. Excessive fibrosis may impair IMDs function, eventually necessitating retrieval or replacement for continued therapy. Therefore, understanding the implant design parameters and their degree of influence on FBR is pivotal to effective and long lasting IMDs. This review gives an overview of FBR as well as anti-FBR strategies. Furthermore, we highlight recent advances in biomimetic approaches to resist FBR, focusing on their characteristics and potential biomedical applications.

|
Scooped by
Beeyond
|
Gao began by noting that prior randomised trials, including SAMMPRIS and VISSIT—as well as initial follow-up data from CASSISS itself, published in 2022—have indicated no benefit when adding PTAS to medical therapy in symptomatic, severe intracranial atherosclerotic stenosis patients. However, he added that it “remains uncertain” whether PTAS can enable improved outcomes at five years or more. With three-year results having already been published, Gao and colleagues’ present analysis of CASSISS sought to shed further light on stenting versus medical therapy in these patients at an even longer follow-up timepoint.

|
Scooped by
Beeyond
|
Endovascular treatments, such as balloon angioplasty alone, balloon-mounted stents, and self-expandable stents, may be of benefit for carefully selected ICAS patients. Two prospective, multicenter, RCT are presently underway to re-evaluate the benefits of endovascular treatments in carefully selected patients (CASSISS trial, and the Wingspan Stent System Post-Market Surveillance Study (WEAVE) trial) (70, 71). These trials' strict selection criteria, identification of stroke mechanisms of intracranial atherosclerosis, as well as use of experienced neurointerventionists in high-volume centers are what makes them of interest for the re-evaluation of invasive ICAS treatment.

|
Scooped by
Beeyond
|
Ceretrieve's mission is to transform stroke care, save lives, minimize disability, and significantly improve post-stroke quality of life. Ceretrieve's aspiration catheter is at the forefront of this mission, delivering aspiration capabilities that are far superior when compared to existing devices, allowing clot(s) removal in a single pass, while fully restoring blood flow. Designed for seamless integration within a conventional 6Fr delivery catheter, Ceretrieve's device offers exceptional trackability and maneuverability, ensuring efficient access to the clot location.

|
Scooped by
Beeyond
|
This study provides Class II evidence that in patients with acute ischemic stroke due to intracranial internal carotid artery (ICA) or M1 segment MCA occlusion, including tandem extracranial ICA occlusions, EVT compared with best medical therapy increased odds of favorable cognitive outcome.

|
Scooped by
Beeyond
|
Researchers at Brigham and Women’s Hospital developed a new test by combining blood-based biomarkers with a clinical score to identify patients experiencing large vessel occlusion strokes (LVO) with high accuracy.

|
Scooped by
Beeyond
|
The μQFR was significantly associated with symptomatic ICAS and outperformed the angiographic stenosis severity. The final nomogram effectively discriminated symptomatic lesions and may provide a useful tool for risk stratification in ICAS patients.

|
Scooped by
Beeyond
|
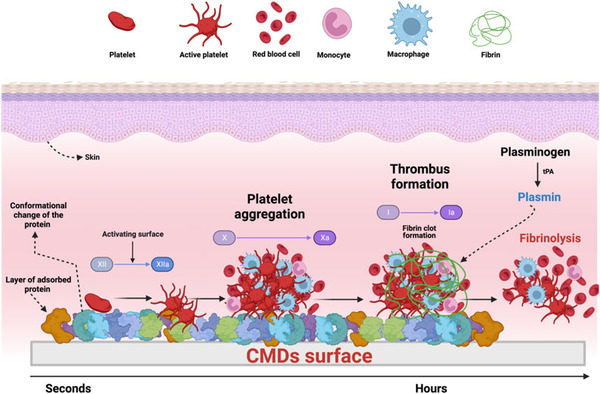
The design of blood-compatible materials requires understanding the physiological mechanisms that give rise to undesirable blood-material interactions. In particular, the materials used in the design of CMDs are determined by the disease for which the CMDs are intended. For example, we review the utility of polymers in developing CMDs when the necessary materials need to be flexible and durable. On the other hand, when there is a need of materials with high mechanical resistance or more remarkable conductors, metals, and metals alloys are the best option. Also, the combination of these two types of materials is used to develop and enhance the ability to perform high workflow rates in CMDs. Even though metals and polymers have tremendous advantages in developing CMDs, there is a predominant recurring challenge, which is biocompatibility.

|
Scooped by
Beeyond
|
Cardiovascular diseases remain the leading cause of death worldwide, with ischemic heart disease (IHD) as the most common. Ischemia-induced angiogenesis is a process in which vascular endothelial growth factor (VEGF) plays a crucial role. To conduct research in the field of VEGF’s association in cardiovascular diseases, it is vital to understand its role in the physiological and pathological processes in the heart. VEGF-based therapies have demonstrated a promising role in preclinical studies. However, their potential in human therapies is currently under discussion. Furthermore, VEGF is considered a potential biomarker for collateral circulation assessment and heart failure (HF) mortality. Additionally, as VEGF is involved in angiogenesis, there is a need to elucidate the impact of VEGF-targeted therapies in terms of cardiovascular side effects.

|
Scooped by
Beeyond
|
Proceeds from the financing will be used to support the clinical and regulatory development of CereVasc's eShunt System, including its upcoming STRIDE pivotal study in patients with Normal Pressure Hydrocephalus. STRIDE is a multi-center, randomized controlled trial that will assess the safety and efficacy of the eShunt System compared to the current standard-of-care ventriculo-peritoneal (VP) shunt and serve as the basis for a future Premarket Approval (PMA) submission to the FDA.

|
Scooped by
Beeyond
|
Scientia Vascular chief commercial officer Paul Fischer said: “We’ve seen what microfabrication can do in products like our Aristotle 24 and Colossus wires in changing the standard of care for patient treatment in stroke, so it’s rewarding to see the excitement and buzz surrounding microfabricated technology in catheters with our physician community.

|
Scooped by
Beeyond
|
Route 92 Medical Inc. is recalling specific lots of Route 92 Medical products containing the Tenzing 7 Delivery Catheters due to multiple instances of distal tip separation at the proximal marker band. The catheters that deconstructed were not manufactured by Route 92 Medical but by an outside contract supplier. Additional investigations determined that some of these catheters manufactured by this outside supplier did not meet Route 92 Medical’s quality standards particularly in the area of the proximal marker band, so a voluntary recall was initiated.

|
Scooped by
Beeyond
|
The reason for the unrivaled flexibility and stability of Plato 17 is the unique micro-machining technology used in Plato 17. Plato 17 has thousands of transition zones to ensure a seamless flexibility gradient: a continuous transition flexibility profile designed to prevent prolapse from selected vessels.Plato 17's unique design: using intricately machined rings and beams on a nickel-titanium tubing (think of it as a 160cm-long nickel-titanium scaffold), a near one-to-one push response is achieved by directly pushing the transition from the proximal to the distal end. This not only prevents kinking, but also helps maintain lumen integrity even in challenging vessels. In sharp contrast, conventional braided and coiled devices tend to accumulate energy, leading to instability and unpredictability

|
Scooped by
Beeyond
|
Although from a commercial point of view coil and Onyx remain the main agents used in clinical practice, it has been observed that in some circumstances they do not guarantee adequate safety and feasibility profiles to maintain human health unaltered. In fact, side effects often occur related to the ease of aggregation, off-target embolisation, collateral circulation, migration and compaction of the spirals, or the presence of solvents such as DMSO (in the case of Onyx), which cause a significant response inflammatory in patients. For this reason, in recent years, scientific research has led to the development of new biomaterials capable of satisfying the growing clinical demands of minimally invasive endovascular embolisation. A variety of embolic materials have been gradually developed, and more research interests have focused on the development of intelligent and/or multifunctional materials. An example is given by embolic agents based on hydrogels, biomaterials of natural or synthetic origin, which are easily customizable with favourable properties and wide versatility.

|
Scooped by
Beeyond
|
CERENOVUS, part of Johnson & Johnson MedTech, has launched CEREGLIDE 71 Aspiration Catheter in Europe, an aspiration catheter equipped with TruCourse technology, indicated for the revascularisation of patients suffering from acute ischemic stroke.

|
Scooped by
Beeyond
|
MIT engineers have developed a magnetically steerable, thread-like robot that can actively glide through narrow, winding pathways, such as the labrynthine vasculature of the brain.

|
Scooped by
Beeyond
|
These findings suggest that low LDL-C levels are associated with an increased bleeding risk within 3 months among patients with minor ischemic stroke or high-risk transient ischemic attack who are receiving dual antiplatelet therapy, especially those taking ticagrelor-aspirin.

|
Scooped by
Beeyond
|
Deployed in the correct manner, future BCIs have the potential to enable their users to control computers with their thoughts after loss of function. This could be life-changing for millions of patients with conditions such as locked-in syndrome, amyotrophic lateral sclerosis (ALS), or tetraplegia. Clinical research and testing of devices for various conditionsopens in a new tab or window is ongoing, and several companiesopens in a new tab or window have made significant stridesopens in a new tab or window to bring these devicesopens in a new tab or window toward commercialization and reaching patients.

|
Scooped by
Beeyond
|
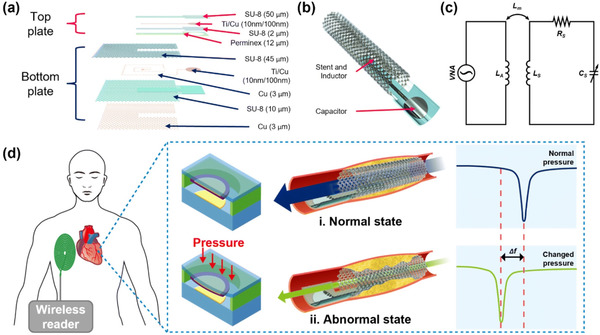
The preliminary findings confirmed the proposed smart stent's higher level of structural integrity, durability and repeatability. Finally, the practical feasibility of the smart stent is demonstrated by monitoring diastole and systole at various beat rates using a phantom. The results of the phantom study showed a similar pattern to the human model, indicating the potential use of the proposed multifunctional smart stent for real-time applications.

|
Scooped by
Beeyond
|
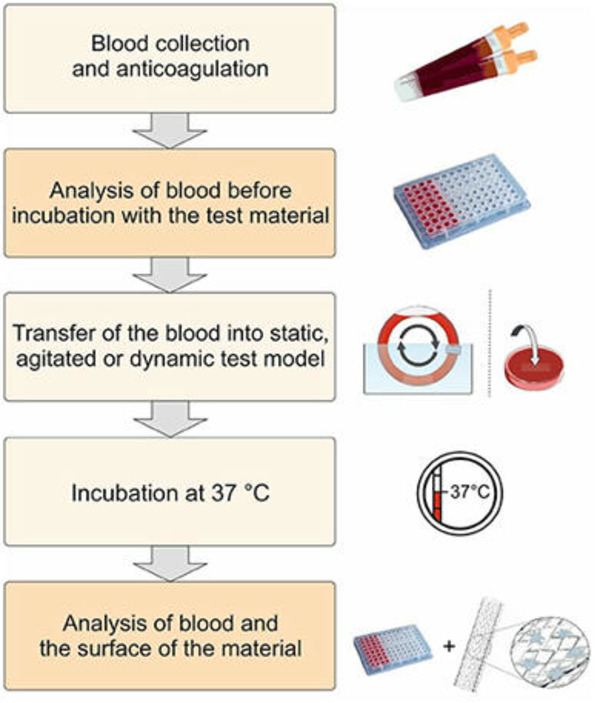
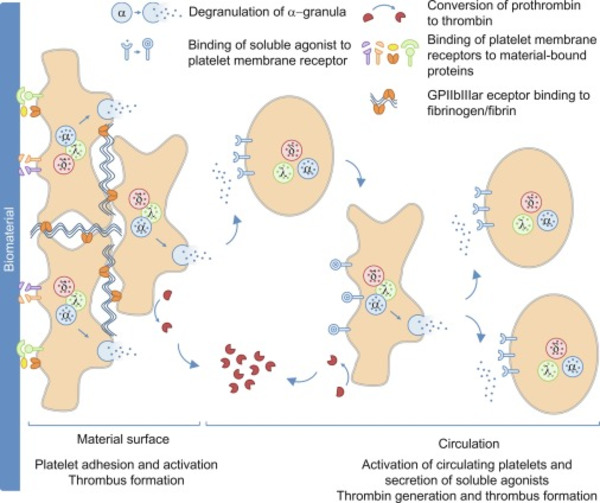
The interaction of biomaterials with blood leads to cellular as well as humoral reactions, which can result in an unwanted inflammation and activation of coagulation and/or fibrinolysis. Thus, the development of biomaterials with an improved hemocompatibility increases the tolerability and minimizes unwanted side effects, such as thrombus formation. Therefore, during the development of new blood-contacting medical devices, not only the mechanical and chemical characteristics should play an important role, but also the hemocompatibility. Furthermore, to prove the safety and reliability of new products, hemocompatibility analyses should include appropriate references and follow the ISO 10993-4 standard.

|
Scooped by
Beeyond
|
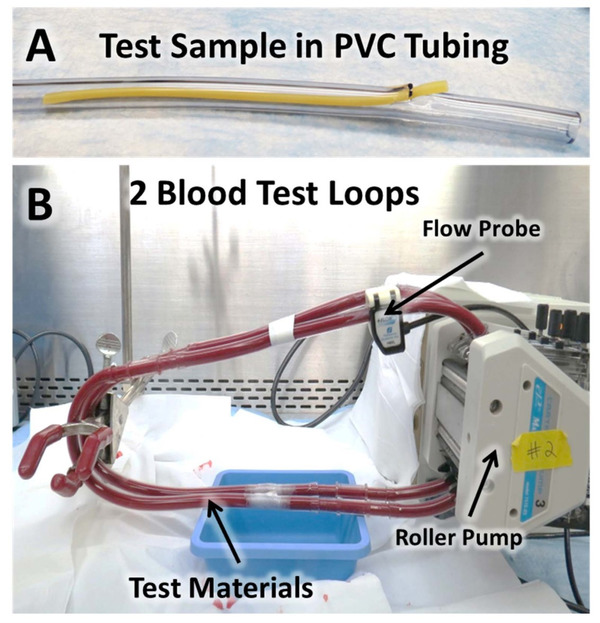
There is currently no standardized test method for in vitro dynamic thrombogenicity assessment of medical devices and biomaterials. This tool will effectively enable users to differentiate device materials with different thrombogenic potentials against standard negative and positive control materials and a marketed comparator device with known thrombogenicity profile.

|
Scooped by
Beeyond
|
These results demonstrated that multiple animal blood sources (particularly donor ovine and bovine blood) may be suitable alternatives to fresh human blood for dynamic thrombogenicity testing when appropriate control materials and donor-specific anticoagulation levels are used.

|
Scooped by
Beeyond
|
To evaluate the use of the large language models (LLMs) GPT-4 and GPT-3.5 to extract data from neuroradiology reports on mechanical thrombectomy in patients with ischemic stroke.

|
Scooped by
Beeyond
|
Since February 2005 when NMPA (then CFDA) published the first batch of medical device clinical sites, a total of 1380 hospitals became certified to conduct clinical research in China as of April 2024. All 1380 sites have different clinical specialties registered with NMPA. We can help you select the right hospital and save time for market introduction.

|
Scooped by
Beeyond
|
In ICAS surgical treatment, when the anterior-posterior diameter difference of the diseased vessel is found to be small and the vessel segment is relatively straight, we can effectively utilize the NOVA® Intracranial Drug-Eluting Stent with the intermediate catheter in place. The use of the NOVA® Intracranial Drug Eluting Stent can significantly simplify the surgical process, reduce the difficulty of the operation, and thus effectively minimize the complications that may arise during the operation. This strategy not only improves the safety of the operation, but also enhances the patient's recovery
|

Curated by Beeyond
BEEYOND is a consulting company in the field of disruptive innovation, accompanying established companies on out-of-the-core growth strategy, from creation of new concepts to product launch. Reach us at: contact@beeyond.fr.
|




 Your new post is loading...
Your new post is loading...